- Resource
- BR1DGE
- Early Detection
- Article
ESPE-ESE 2025 Symposium Summary
At the BR1DGE symposium at ESPE-ESE 2025, experts discussed the benefits of early detection of people with presymptomatic type 1 diabetes (T1D) and the practical insights from ongoing T1D screening programs.
This article is intended to be a summary only. Additional topics were covered in the live symposium in line with the applicable regulations but are not included on the BR1DGE platform.
Meeting Objectives
- Explore the benefits of early detection of people with presymptomatic T1D
- Discuss practical insights from ongoing T1D screening programs
Speakers
A Global Perspective: The Power of Early Detection in T1D
“Sreening is now a global initiative which can help early detection of T1D through multiple antibodies and would help individuals for softlanding in diabetes treatment.”
In the opening presentation, Prof. Thomas Danne discussed the importance of early detection of T1D, emphasising the benefits of identifying pre-symptomatic individuals.
- Early detection helps diagnosis of T1D and reduces significant burden and risk of diabetic ketoacidosis (DKA)1-7and T1D related complications. Long-term consequences of DKA at diagnosis is associated with increased morbidity and mortality,8,9 negative impacts glycemic control,8 and is associated with neurocognitive deficits.10-12
- Early detection provides an opportunity to develop glycaemic skills and psychologically prepare individuals with T1D, reducing stress at the time of diagnosis13
- Screening for the presence of ≥ 2 islet autoantibodies (IAbs) can identify individuals with presymptomatic T1D and can initiate personalised treatment depending on age14, IAbs number15-19 and type14,18-20
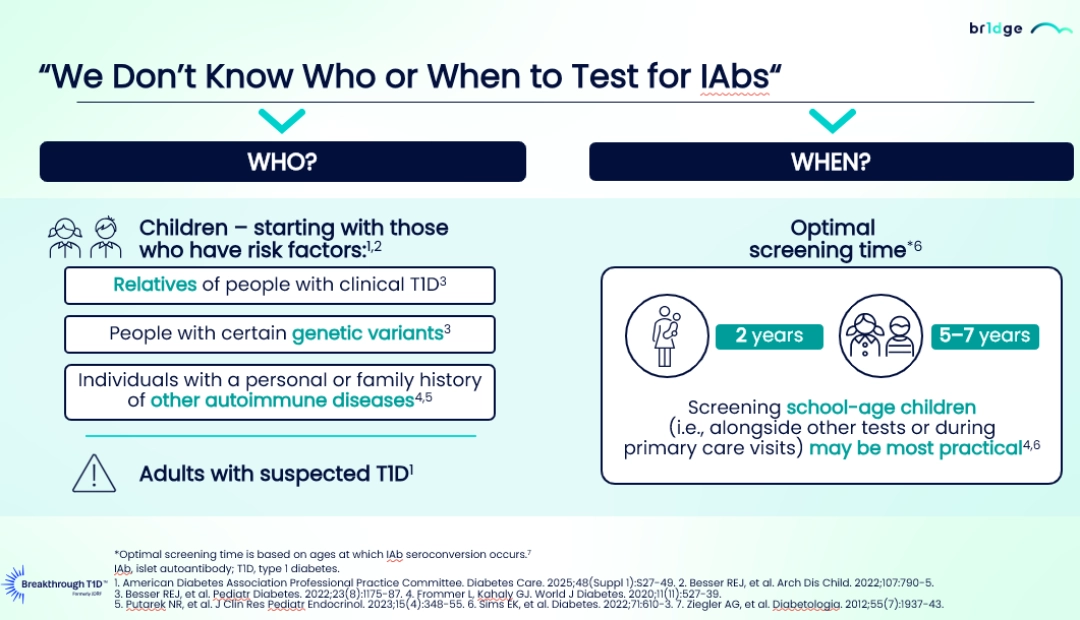
- Screening school-age children (5-7 years)21-23 during primary care visits, as well as adults with risk factors, such as relatives of people with clinical T1D,24 specific genetic variants,24 or a family history of other autoimmune diseases21,22 represents the most practical approach.
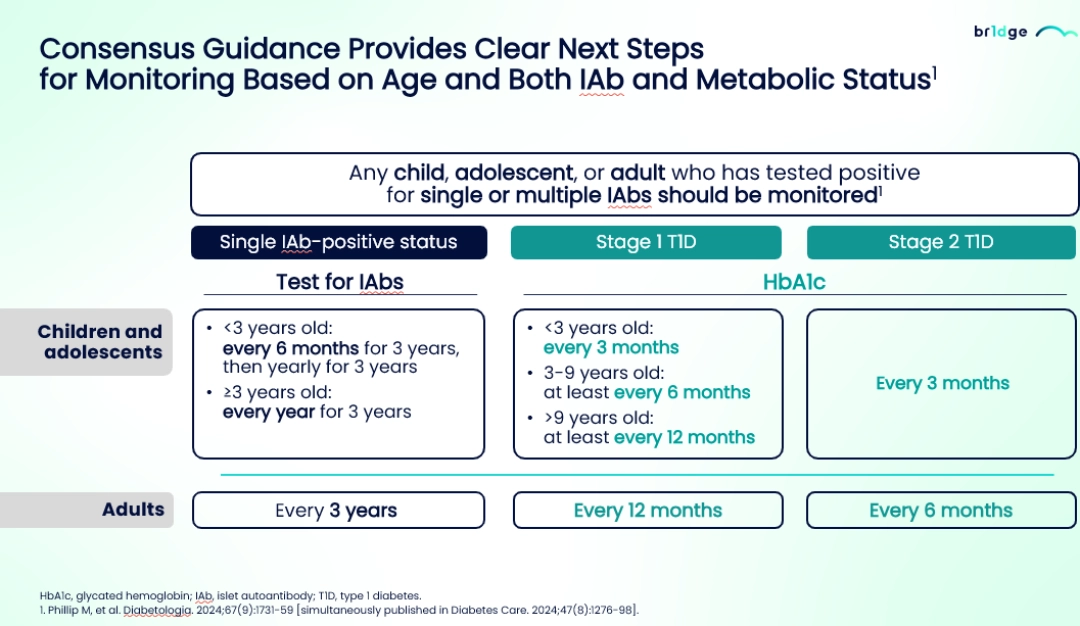
- The international consensus guidelines recommends that healthcare professionals (HCPs) to provide individuals who are diagnosed positive for IAbs results with a comprehensive management approach including educational materials, psychological assistance, and tools for metabolic monitoring. The recommended frequency of monitoring of autoantibodies should be tailored based on the individual's IAbs profile, metabolic status, and age16
Local Action, Global Impact: Conversations on Screening Programs in T1D
“We really have to make sure that the families we are screening understand exactly why we are doing this. People are more likely to take up the idea if they understand why... it is a challenging thing to describe to people who do not have any personal connection to the disease.”
Panel Discussion: Flemming Pociot, Thomas Danne and Michael J. Haller
Profs. Flemming Pociot, Thomas Danne, and Michael J. Haller discussed the practical insights from the ongoing T1D screening programs.
- The panel emphasised the need for clear and effective communication with families about the purpose and benefits of screening and awareness on T1D management.1,2
- The consensus provides clear guidance regarding the monitoring stating that children, adolescents or adults who have tested positive for single or multiple IAbs should be monitored based on age and both IAbs and metabolic status and the monitoring frequency should be more often for young children than the adults.3
- Prof. Danne provided an overview of various technologies currently available and highlighted the use of continuous glucose monitoring (CGM) in monitoring disease progression of T1D and introduced time in normoglycaemia, a new CGM metric.3
- Early identification of presymptomatic T1D may reduce the psychological burden3 experienced at diagnosis of clinical (Stage 3) diabetes by giving time for preparation and offer support and education.4,5
- The panelists advocated for widespread population screening, citing the prevalence of T1D and recommended to discuss the staging of T1D with local clinicians and create awareness on presymptomatic stages and screening programs.
At the time of this presentation, Flemming Pociot declared the following conflicts of interest: Research funding: Sanofi; Speaker/Consultant: Sanofi.
At the time of this presentation, Thomas Danne declared the following conflicts of interest: Foundation: Chief Medical Officer of Breakthrough T1D (previously JDRF); Speaker/Consultant: AstraZeneca, Abbott, Bayer, Biomeia, Boehringer, Dexcom, DreaMed Diabetes, Ltd., Eli Lilly, Insulet, Medtronic, Menarini, Novo Nordisk, Roche, Sanofi, Vitalaire, Ypsomed.
At the time of this presentation, Michael J. Haller declared the following conflicts of interest: Research funding: Mannkind, Sanofi, SAB BIO; Speaker/Consultant: Mannkind, Sanofi; Stocks/Shareholder: SAB BIO.
MAT-GLB-2503072–1.0–06/2025
What Does the Future of Islet Autoantibody Screening Technology Look Like?
- Shrivastava SR, et al. J Diabetes Metab Disord. 2013;12:14.
- Smudja M, et al. PLoS One. 2024;19(3):e0300055.
- Ventura AD, et al. Diabetes MILES-2 2016 Survey Report. Melbourne, AU: Diabetes Victoria; 2016. https://acbrd.org.au/wp-content/uploads/2018/06/C292_DV_miles-2_web_final.pdf. Accessed May 2025.
- Wolfsdorf JI, et al. Pediatr Diabetes. 2018:19 Suppl 27:155-77.
- Usher-Smith JA, et al. Diabetologia. 2012;55(11):2878-94.
- Birkebaek NH, et al. Lancet Diabetes Endocrinol. 2022;10(11):786-94.
- Cherubini V, et al. Diabetologia. 2020;63:1530-41.
- Duca LM, et al. Diabetes Care. 2017;40(9):1249-55.
- Duca LM, et al. Pediatr Diabetes. 2019;20:172-9.
- Aye T, et al. Diabetes Care. 2019;42(3):443-9.
- Cameron FJ, et al. Diabetes Care. 2014;37(6):1554-62.
- Ghetti S, et al. Diabetes Care. 2020;43:2768-75.
- Besser REJ, et al. Arch Dis Child. 2022;107:790–5.
- Jacobsen LM, et al. Diabetologia. 2020;63(3):588-96.
- Insel RA, et al. Diabetes Care. 2015;38(10):1964-74.
- Phillip M, et al. Diabetologia. 2024;67(9):1731-59 [simultaneously published in Diabetes Care. 2024;47(8):1276-98].
- Anand V, et al. Diabetes Care. 2021;44(10):2269-76.
- So M, et al. Endocr Rev. 2021;42(5):584-604.
- Pöllänen PM, et al. Diabetologia. 2017;60(7):1284-93.
- Suomi T, et al. EBioMedicine. 2023;92:104625.
- Frommer L, Kahaly GJ. World J Diabetes. 2020;11(11):527-39.
- Putarek NR, et al. J Clin Res Pediatr Endocrinol. 2023;15(4):348-55.
- Sims EK, et al. Diabetes. 2022;71:610-3.
- Besser REJ, et al. Pediatr Diabetes. 2022;23(8):1175-87.
Local Action, Global Impact: Conversations on Screening Programs in T1D
- Hendriks AEJ, et al. Diabetes Metab Res Rev. 2024;40(2):e3777.
- Simmons KMW, et al. Diabetes Technol Ther. 2023;25(11):790-9.
- Phillip M, et al. Diabetologia. 2024;67(9):1731-59 [simultaneously published in Diabetes Care. 2024;47(8):1276-98].
- Besser REJ, et al. Arch Dis Child. 2022;107:790-5.
- Smith LB, et al. Pediatr Diabetes. 2018;19(5):1025-33.