- Resource
- BR1DGE
- Disease Burden
- Pathophysiology
- Staging
The Importance of Early T1D Detection
Learn about autoimmune type 1 diabetes (T1D) and the importance of early detection through islet autoantibody (IAb) screening.
.png/jcr:content/Thumbnail_1190x500%20(1).png)
Learning Objectives
- Recognize the burden of T1D for individuals and families
- Describe the four distinct stages of T1D, including the significance of IAbs in the presymptomatic stages
- Explain the benefits of early identification of T1D
The Importance of Early T1D Detection
Our understanding of T1D is changing. Screening for islet autoantibodies (IAbs) can now identify individuals who are at risk of developing T1D long before the onset of clinical symptoms.1 This early identification gives people time to prepare for the clinical onset of the disease, with potential benefits for both physical and mental health.2–4
Many people discover they have T1D only when symptoms appear
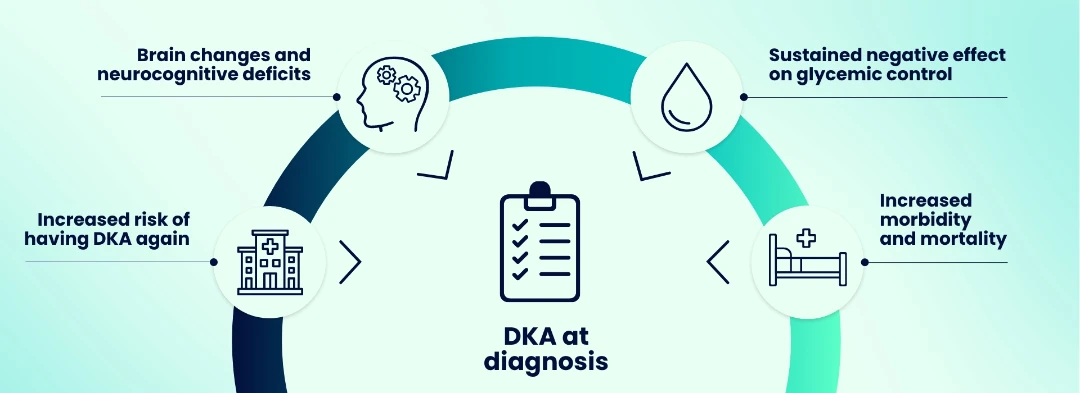
Diagnosis of T1D (Stage 3) often occurs in hospital, with the presentation of severe hyperglycemia and associated symptoms.5–8 People may also present with diabetic ketoacidosis (DKA)—an acute, potentially life-threatening metabolic complication that is associated with serious short- and long-term consequences.9–14
Younger age of onset of T1D is associated with reduced life expectancy and increased risk of DKA.15–18 In addition, lower socioeconomic status and the presence of mental illness or distress are associated with suboptimal glycemic control and increased risk of DKA.19–21
The long-term impacts of DKA at diagnosis of Stage 3 T1D include:12–14,22–24
A diagnosis of T1D may have a significant impact on individuals, caregivers, and families
Individuals diagnosed with T1D must rapidly adapt to the realities of living with the disease. Challenges include:
- The complexities of daily disease management25
- Negative impacts on finances, education, work, mental health, and family life26–29
- Complications associated with hyperglycemia and hypoglycemia15,22
In addition, parents or caregivers of children with T1D may experience worry and psychological distress related to their child’s disease.30
T1D has a presymptomatic phase with characteristic biomarkers
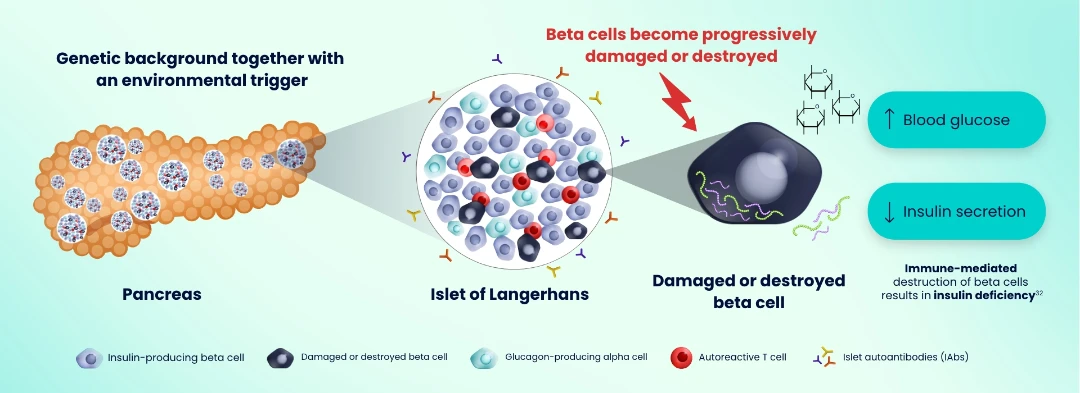
Evidence suggests that genetic and environmental factors combine to trigger the autoimmune process that drives T1D. Activated autoreactive T cells progressively damage and destroy pancreatic insulin-producing beta cells, resulting in insulin deficiency.31–33 Autoantigens from damaged beta cells trigger the production of IAbs — critical biomarkers of the autoimmune process that drives the disease.1,31
Autoreactive T cells drive progressive damage and destruction of insulin-producing beta cells5,31–34
Screening for IAbs allows early identification of individuals who are at risk of developing T1D
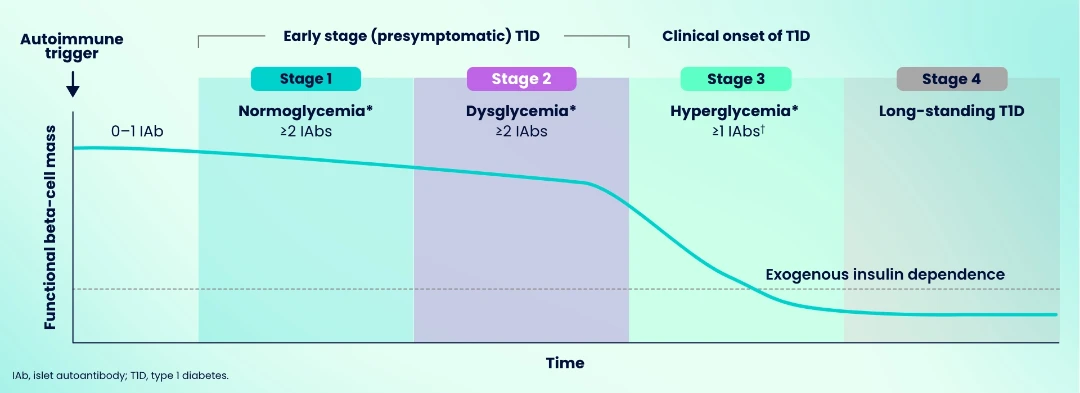
Presymptomatic T1D is defined by the persistent presence of ≥2 IAbs together with either normoglycemia (Stage 1 T1D) or dysglycemia (Stage 2 T1D).1,35 These IAbs can be detected months or years before the clinical onset of disease (Stage 3 T1D).5,32 Individuals with single-IAb positivity are at risk of developing early-stage T1D and require monitoring for disease progression.1,36 Multiple-IAb positivity (≥2 IAbs) indicates a near 100% lifetime risk of developing Stage 3 T1D in children.35
T1D progresses in four distinct stages, linked to functional beta-cell mass1,5,35,37–40
*Normoglycemia: FPG <100 mg/dL(<5.6 mmol/l); dysglycemia: FPG 100–125 mg/dL (5.6–6.9 mmol/l); hyperglycemia: FPG ≥126 mg/dL (>7.0 mmol/l).36 †IAbs may become absent.1
Early identification of T1D may benefit affected individuals and their family members or caregivers
Potential benefits of early identification of T1D include:2–4,40–42
- Reduced risk of DKA and hospitalization at the onset of Stage 3 T1D
- Reduced psychological impacts
- An opportunity to provide education, counseling, and support, which has been shown to improve clinical and quality-of-life outcomes
After an individual has screened positive for IAbs and is diagnosed with Stage 1 or 2 T1D, regular assessments of blood glucose levels are recommended as part of a strategy to monitor progression. Multiple metabolic assessment tools are available to monitor disease progression. Breakthrough T1D™ (formerly the Juvenile Diabetes Research Foundation [JDRF]) has published consensus guidance on monitoring (including the choice of metabolic assessment tools and frequency of visits).36
Abbreviations
DKA, diabetic ketoacidosis; FPG, fasting plasma glucose; IAb, islet autoantibody; T1D, type 1 diabetes.
References
- ADA. Diabetes Care. 2025;48(Suppl. 1):S27-49.
- Greenbaum CJ, et al. Diabetes. 2021;70(5):1029-37.
- Hummel S, et al. Diabetologia. 2023;66:1633-42.
- Smith LB, et al. Pediatr Diabetes. 2018;19(5):1025-33.
- DiMeglio LA, Evans-Molina C, Oram RA. Lancet. 2018;391(10138):2449-62.
- Dayan CM, et al. Science. 2021;373(6554):506-10.
- Hendriks AEJ, et al. Diabetes Metab Res Rev. 2024;40(2):e3777.
- Muñoz C, et al. Clin Diabetes. 2019;37(3):276-81.
- Dhatariya KK, et al. Nat Rev Dis Primers. 2020;6(1):40.
- Ehrmann D, et al. Lancet Diabetes Endocrinol. 2020;8(5):436-46.
- Gibb FW, et al. Diabetologia. 2016;59:2082-7.
- Aye T, et al. Diabetes Care. 2019;42:443-9.
- Ghetti S, et al. Diabetes Care. 2020;43(11):2768-75.
- Cameron FJ, et al. Diabetes Care. 2014;37:1554-62.
- Rawshani A, et al. Lancet. 2018;392:477-86.
- Rugg-Gunn CEM, et al. JAMA Pediatr. 2022;176(12):1248-59.
- Praveen PA, et al. Pediatric Diabetes. 2022;22(1):40-6.
- Karavanaki K, et al. Hormones (Athens). 2024;23(3):395-405.
- Weinstock RS, et al. J Clin Endocrinol Metab. 2013;98(8):3411-9.
- Hong KMC, et al. Acta Diabetol. 2021;58(12):1627-35.
- Hernar I, et al. Diabetes Care. 2024;47(1):126-31.
- Duca LM, et al. Diabetes Care. 2017;40(9):1249-55.
- Hammersen J, et al. Pediatr Diabetes. 2022;22(3):455-62.
- Duca LM, et al. Pediatr Diabetes. 2019;20:172-9.
- Shrivastava SR, et al. J Diabetes Metab Disord. 2013;12:14.
- Dehn-Hindenberg A, et al. Diabetes Care. 2021;44(12):2656-63.
- Butwicka A, et al. Diabetes Care. 2015;38:453-9.
- Fleming M, et al. Diabetes Care. 2019;42(9):1700-7.
- Nielsen HB, et al. Diabetes Res Clin Pract. 2016;121:62-8.
- Whittemore R, et al. Diabetes Educ. 2012;38(4):562-79.
- Powers AC. J Clin Invest. 2021;131(8):e142242.
- Pugliese A. Pediatr Diabetes. 2016;17(Suppl 22):31-6.
- Clark M, et al. Front Immunol. 2017;8:1898.
- Houeiss P, Luce S, Boitard C. Front Endocrinol (Lausanne). 2022;13:933965.
- Insel RA, et al. Diabetes Care. 2015;38(10):1964-74.
- Phillip M, et al. Diabetologia. 2024;67(9):1731-59 [simultaneously published in Diabetes Care. 2024;47(8):1276-98].
- Buzzetti R, et al. Nat Rev Dis Primers. 2022;8(1):63.
- Redondo MJ, et al. Diabetes Care. 2018;41(2):311-7.
- Redondo MJ, et al. J Clin Endocrinol Metab. 2020;105(5):1629-40.
- Besser REJ, et al. Pediatr Diabetes. 2022;23(8):1175-87.
- Steck AK, et al. Pediatr Diabetes. 2017;18(8):794-802.
- Scheiner G, et al. ADCES in Practice. 2022;10(5):20-5.
©Sanofi 2025 - All Rights Reserved. MAT-GLB-2405367-1.0 - 07/2025
